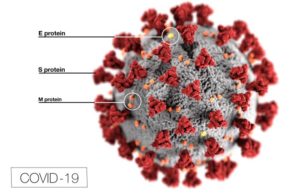
COVID-19 illustration by the Centers for Disease Control and Prevention.
Pfizer is asking U.S. regulators to allow boosters of its COVID-19 vaccine for anyone 18 or older.
Older Americans and other groups particularly vulnerable to the virus have had access to a third dose of the Pfizer and BioNTech vaccine since September. But the Food and Drug Administration has said it would move quickly to expand boosters to younger ages if warranted.
The filing was announced Tuesday.
Pfizer is submitting early results of a booster study in 10,000 people to make its case that it’s time to further expand the booster campaign.
| By LAURAN NEERGAARD, The Associated Press |
























